There you go...that sounds like a marketable name.Another update:
The current project code name is ION HAMMER.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
There you go...that sounds like a marketable name.Another update:
The current project code name is ION HAMMER.
Another update:
The current project code name is ION HAMMER.
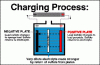

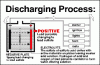

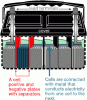
We used 500 lb 2V cells for the 48V UPS in our computer rooms, which were very high quality. The clear case to inspect the sediment and plates and cell S.G. balance was important. ( Telephone companies must remove/recycle these whenever they become mismatched even if they still have good life left. ) ( hint)
We only had problems with the emergency light batteries as I recall which were conventional small deep discharge types. We had Liebert UPS units.
I recall it was the antimony dendrites that can chain to short plates once the separator has worn. I experienced this effect once with a car in 1970. One second, it wouldn't turn over. then next time it started like new, thus disturbing the chain from the first attempt and allowing it to settle. Then repeating each day or so with occasional shorts causing outgassing until battery replaced.
You can inspect the health of the acid particulate contamination with a laser pointer and look for sparkles floating in acid. THe conductive particles will likely sparkle and the non-conductive particles will reflect light but more diffused.
