hello
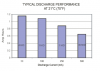
i saw alot of flashlight that uses 3*aaa alkaline(primary cell)batteries in series,for running the led with 1A,1.5A (and even more )current.and i read,also,that this cell doesn't like currents above 0.5C(more that around 600mA,when it's capacity is 1200mAh)so how can this battery can handle 1500mA currents?(cells in series doesn't increase the capacity,and therefore not have the ability to divide the high current between them,still will have 1200mAh)so aren't these current won't kill the battery?is it ok. and normal to use that currents on these 3*aaa bateries when running a flashlight?and if not,why to sell a flashlight with currents that the battery can't handle?
what should be,really,the maximum current that we can load on the 3 alkaline batteries in series to operate a flashlight,and still to have the ability to enjoy from the use of the flashlight,without kill the battery?(what is the maximum current that allowed to consume from them?)
thanks in advance.
i saw alot of flashlight that uses 3*aaa alkaline(primary cell)batteries in series,for running the led with 1A,1.5A (and even more )current.and i read,also,that this cell doesn't like currents above 0.5C(more that around 600mA,when it's capacity is 1200mAh)so how can this battery can handle 1500mA currents?(cells in series doesn't increase the capacity,and therefore not have the ability to divide the high current between them,still will have 1200mAh)so aren't these current won't kill the battery?is it ok. and normal to use that currents on these 3*aaa bateries when running a flashlight?and if not,why to sell a flashlight with currents that the battery can't handle?
what should be,really,the maximum current that we can load on the 3 alkaline batteries in series to operate a flashlight,and still to have the ability to enjoy from the use of the flashlight,without kill the battery?(what is the maximum current that allowed to consume from them?)
thanks in advance.