while reading through https://archive.org/download/Chemis...ics-_Basic_Principles_and_Theory_Conkling.pdf because tomorrow is the 4th of July, and it's become a family tradition that i make some of the fireworks, i came across a table of "photoflash" mixtures in this chart:
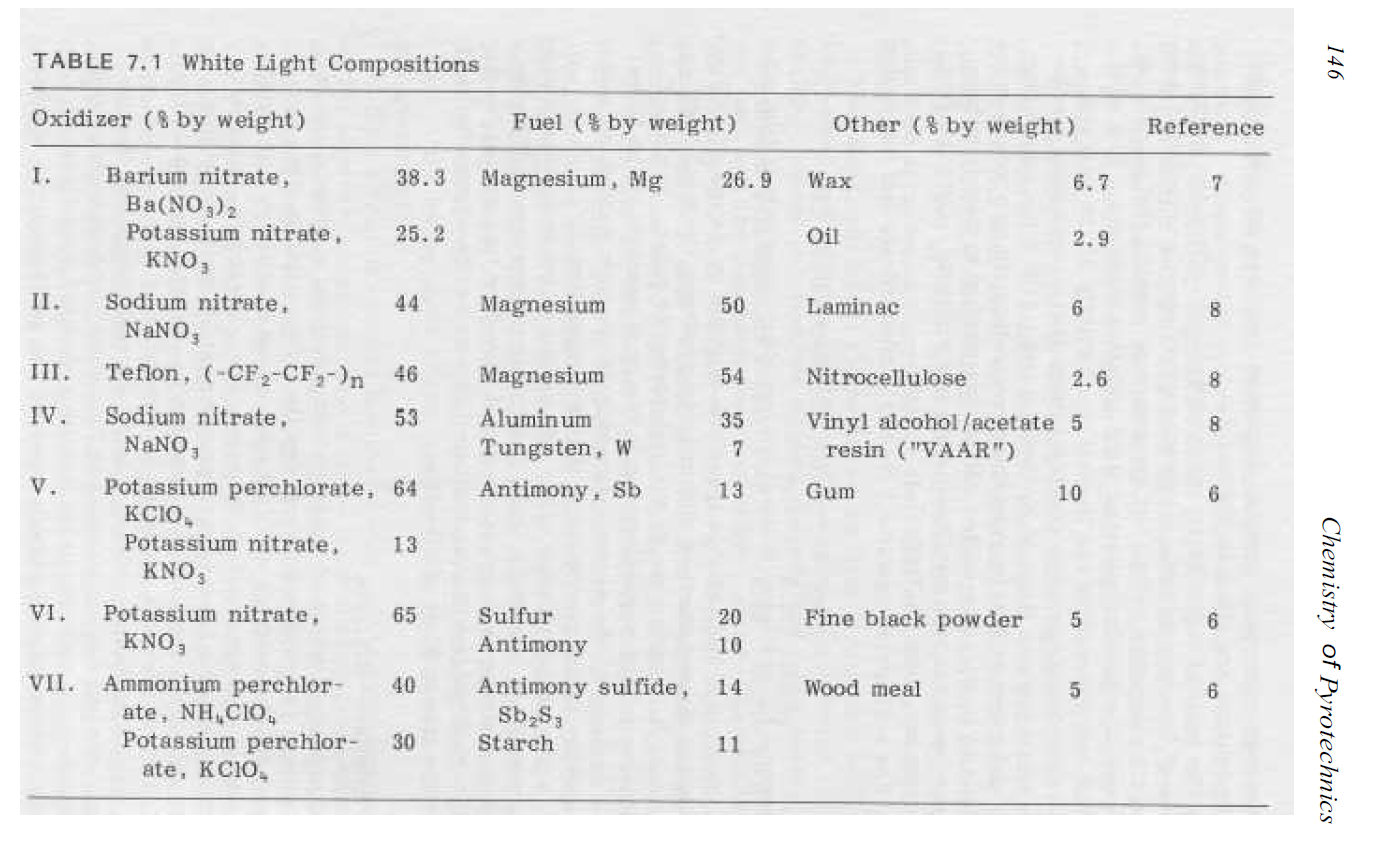
i thought i was seeing things, but the third compound uses Teflon as the oxidizer (in this case it's fluorine (F) that is the oxidizing element, and fluorine is the most reactive of the halogens). i've always considered Teflon to be stable and mostly inert, although it does have a habit of poisoning soldering iron tips. it's the same release of fluorine at high temperatures that makes it an oxidizer in pyrotechnic compounds. one potential hazard when using teflon in a mixture is that due to it's electrical insulating properties, Teflon can take on static charges very easily, and when mixing pyrotechnic compounds that's the LAST thing you want.
i thought i was seeing things, but the third compound uses Teflon as the oxidizer (in this case it's fluorine (F) that is the oxidizing element, and fluorine is the most reactive of the halogens). i've always considered Teflon to be stable and mostly inert, although it does have a habit of poisoning soldering iron tips. it's the same release of fluorine at high temperatures that makes it an oxidizer in pyrotechnic compounds. one potential hazard when using teflon in a mixture is that due to it's electrical insulating properties, Teflon can take on static charges very easily, and when mixing pyrotechnic compounds that's the LAST thing you want.

