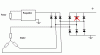
The diode, that I red crossed, died (went to short circuit).
I replaced the diodes and now everything is OK.
What I am trying to understand is what exactely was going on
when the circuit was malfunctioning that made the battery
boil.
Any help is welcome.
Thanks.
I replaced the diodes and now everything is OK.
What I am trying to understand is what exactely was going on
when the circuit was malfunctioning that made the battery
boil.
Any help is welcome.
Thanks.