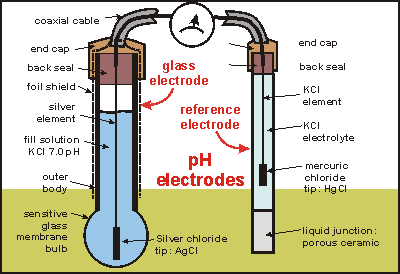
I am trying to learn how a battery powered PH meter works?
I have 4 different home made red wines, a total of 18 gallons and 11 more gallons to make. Ph paper is not accurate to .1 measurements. It is hard to read 1/2 Ph with ph paper.
People on the wine forum say, buy a good $50 Ph meter. People that have already bought ph meters say, $10, $20, $30, ph meters don't work. Some people with $60 meters claim $60 ph meters don't work.
I assume a ph meter tests the ohm resistance of the acid in the wine. Factory wine is usually 3ph to 4 ph. I made a 1" gap with 2 wires then tested tap water and 2 different factory made wines and 1 of my home made wines.
City water = 96K ohms
The Cab wine = 83.6K ohms ph paper red
The Dark wine = 74.6K ohms ph paper less red
As you can see ph paper is very hard to read. I can barely see the difference in the 2 red ph paper ends.
People on the wine forum don't know about electricity. Does anyone here make wine?
I don't have a digital meter or old needle meter to show ohms for 1" gap wire but I don't need one. I like the flavor of the 74.6K ohm wine best, I think I can add citric acid to wine to get 74.6K ohms. Wine will not be ready to bottle for 6 months nothing to do but wait.
I think I am on the right track. What do you think?
Does anyone know how a battery ph meter works?
I did a search for ph meter circuits and found none.






I have 4 different home made red wines, a total of 18 gallons and 11 more gallons to make. Ph paper is not accurate to .1 measurements. It is hard to read 1/2 Ph with ph paper.
People on the wine forum say, buy a good $50 Ph meter. People that have already bought ph meters say, $10, $20, $30, ph meters don't work. Some people with $60 meters claim $60 ph meters don't work.
I assume a ph meter tests the ohm resistance of the acid in the wine. Factory wine is usually 3ph to 4 ph. I made a 1" gap with 2 wires then tested tap water and 2 different factory made wines and 1 of my home made wines.
City water = 96K ohms
The Cab wine = 83.6K ohms ph paper red
The Dark wine = 74.6K ohms ph paper less red
As you can see ph paper is very hard to read. I can barely see the difference in the 2 red ph paper ends.
People on the wine forum don't know about electricity. Does anyone here make wine?
I don't have a digital meter or old needle meter to show ohms for 1" gap wire but I don't need one. I like the flavor of the 74.6K ohm wine best, I think I can add citric acid to wine to get 74.6K ohms. Wine will not be ready to bottle for 6 months nothing to do but wait.
I think I am on the right track. What do you think?
Does anyone know how a battery ph meter works?
I did a search for ph meter circuits and found none.
Last edited: