First the disclaimer.....
yes this is a HHO cell made to produce gas, no it isnt a over unity device. Its function is to produce nice clean gases that are separated and used for something else. It has nothing to do with engines etc! The other disclaimer as you will see is this is from a decent Journal and the article is actually about the catalyst used.
I have use for both on demand Hydrogen and Oxygen, easy enough to do electrically but you always run into the same problems sooner or later. I could get cylinders but there is a rental charge and they are getting expensive to have delivered, also sometimes like this time I need constant but small amounts.
Normally i go the smidge of Sulphuric acid in DI water router, electrode in glass tubes similar to a hoffman set up used to prove that water is made from Hydrogen and Oxygen. The cheaper you can make the gas the better, the less faffing with drying the gasses etc the better. The OU boys swear by Tungsten as an electrode but its rubbish! Now its proven rubbish by this journal.
The obvious choices for electrodes that last and dont contaminate would be platinum, can you spot the problem with using that? . But turns out apparently not. Reading Febs Journal of chemical education I came across a demo in the journal for splitting water using cheap to get top class electrodes of decent size!
. But turns out apparently not. Reading Febs Journal of chemical education I came across a demo in the journal for splitting water using cheap to get top class electrodes of decent size!
Your welcome to read the abstract which has alot of info, i wont post the entire pdf because of copywrite and loosing my access to the uni library!
https://pubs.acs.org/doi/pdf/10.1021/acs.jchemed.7b00537
But if you want a full copy for personal use get in touch.
The article goes into the problems of over potential etc etc and gives various figures for Tungsten and other electrodes. The main difference is this cell is an alkali cell using Sodium Hydroxide, so that cuts out alot of metal electrodes for a start.
Using Hydroxide is actually pretty clever, it oxidizes really well but is rough on most metals. those metals its not rough on tend not to be efficient. For Oxidizing the OH- ions at the anode they used plates made from an alloy containing roughly 1.69%cobalt, 40.44% chromium, and 7.87% platinum. At 1M concentration of Sodium Hydroxide you would see zero wear at the anode, i got to be honest and say with nearly 8% platinum and cobalt, these plates might also be able to handle chlorate production.
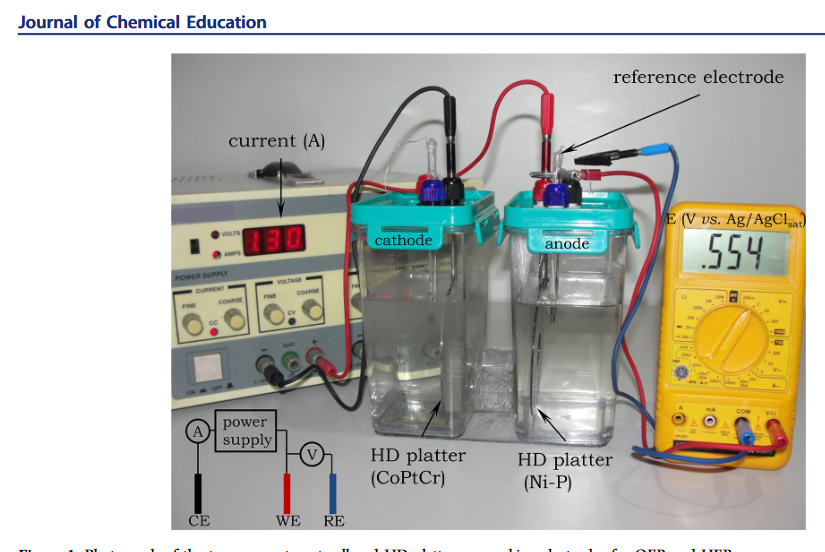
Anyway for producing Hydrogen, water is reduced At the cathode using a Nickel−phosphorus plate, the two cells are bridged.
Now for the best bit of all, where do you get these catalytic super electrodes? Turns out the top plate of a HDD is made with a coating of CoPtCr and the under side is made with a coating of Nickel−Phosphorus . here is a screen shot from the journal article.
. here is a screen shot from the journal article.
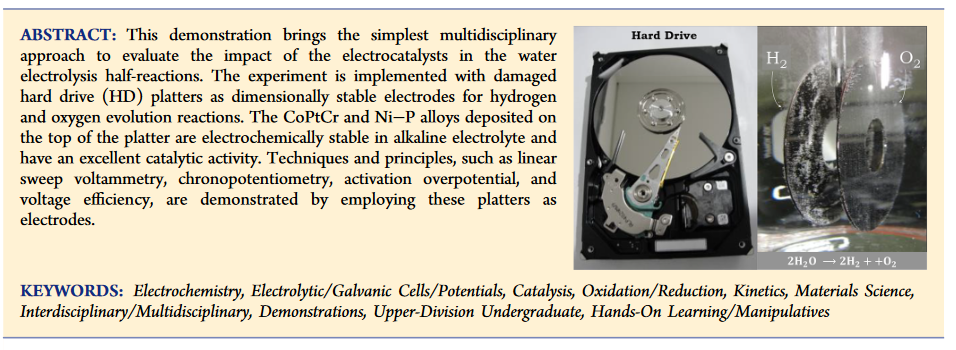
I suspect this applies mainly to older plates considering the amount of platinum in them, but having never really thought about what the plates were made from, might be worth using them for other things. Anyway if anyone does want the complete article pm me.
P.S
So far i have used 3M hydroxide and absolutely no pitting! You get alot more gas by using Hydroxide to most other methods.
yes this is a HHO cell made to produce gas, no it isnt a over unity device. Its function is to produce nice clean gases that are separated and used for something else. It has nothing to do with engines etc! The other disclaimer as you will see is this is from a decent Journal and the article is actually about the catalyst used.
I have use for both on demand Hydrogen and Oxygen, easy enough to do electrically but you always run into the same problems sooner or later. I could get cylinders but there is a rental charge and they are getting expensive to have delivered, also sometimes like this time I need constant but small amounts.
Normally i go the smidge of Sulphuric acid in DI water router, electrode in glass tubes similar to a hoffman set up used to prove that water is made from Hydrogen and Oxygen. The cheaper you can make the gas the better, the less faffing with drying the gasses etc the better. The OU boys swear by Tungsten as an electrode but its rubbish! Now its proven rubbish by this journal.
The obvious choices for electrodes that last and dont contaminate would be platinum, can you spot the problem with using that?
Your welcome to read the abstract which has alot of info, i wont post the entire pdf because of copywrite and loosing my access to the uni library!
https://pubs.acs.org/doi/pdf/10.1021/acs.jchemed.7b00537
But if you want a full copy for personal use get in touch.
The article goes into the problems of over potential etc etc and gives various figures for Tungsten and other electrodes. The main difference is this cell is an alkali cell using Sodium Hydroxide, so that cuts out alot of metal electrodes for a start.
Using Hydroxide is actually pretty clever, it oxidizes really well but is rough on most metals. those metals its not rough on tend not to be efficient. For Oxidizing the OH- ions at the anode they used plates made from an alloy containing roughly 1.69%cobalt, 40.44% chromium, and 7.87% platinum. At 1M concentration of Sodium Hydroxide you would see zero wear at the anode, i got to be honest and say with nearly 8% platinum and cobalt, these plates might also be able to handle chlorate production.
Anyway for producing Hydrogen, water is reduced At the cathode using a Nickel−phosphorus plate, the two cells are bridged.
Now for the best bit of all, where do you get these catalytic super electrodes? Turns out the top plate of a HDD is made with a coating of CoPtCr and the under side is made with a coating of Nickel−Phosphorus
I suspect this applies mainly to older plates considering the amount of platinum in them, but having never really thought about what the plates were made from, might be worth using them for other things. Anyway if anyone does want the complete article pm me.
P.S
So far i have used 3M hydroxide and absolutely no pitting! You get alot more gas by using Hydroxide to most other methods.
