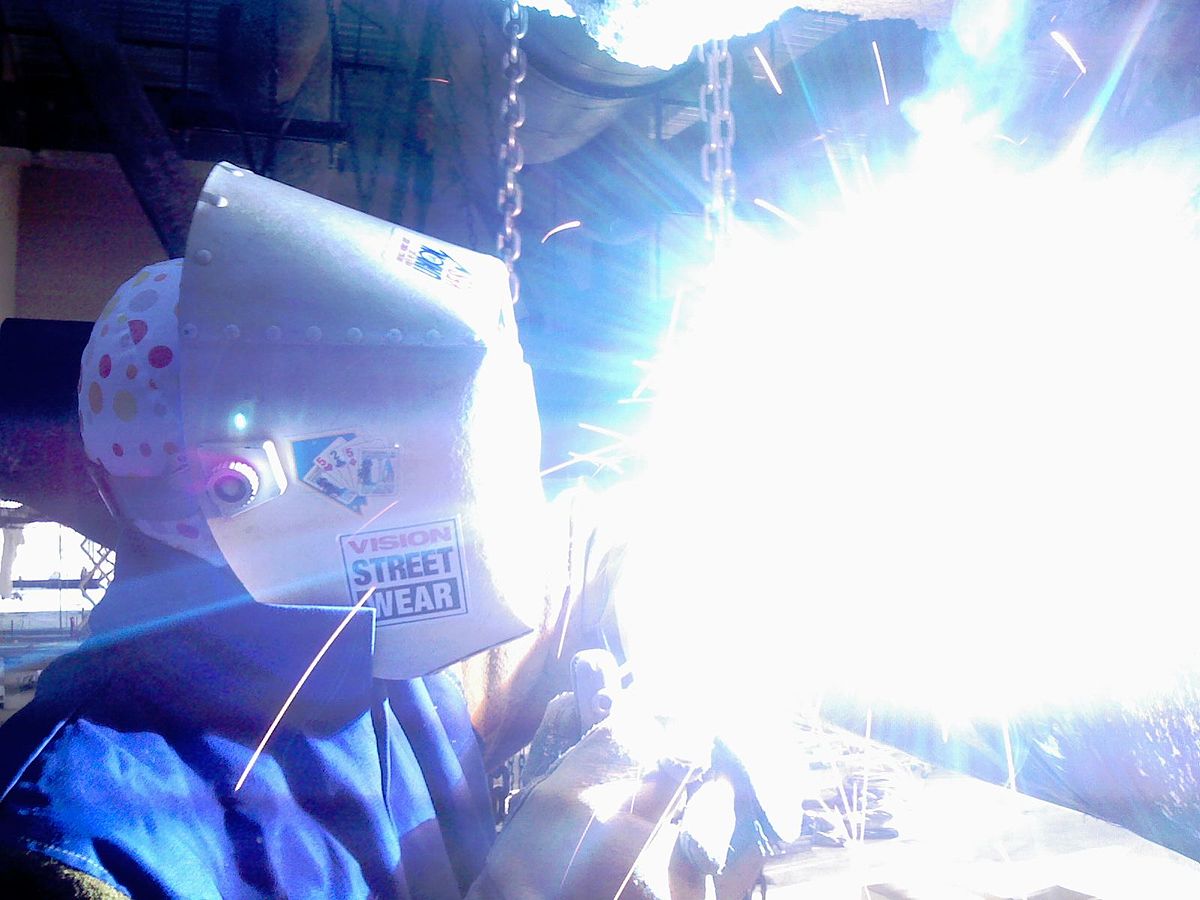
I want to make an ultraviolet generator based on a spark gap in air. I tried using a drill motor as this.
To do this, I covered the contacts on the commutator with a marker to make the contact worse. Supplied 19.8 V from laptop charging, serial connection of electromagnets with rotor windings, 15 ohms. The current was about 1A.
After about 20 minutes of rotation of the engine, the brushes were worn off.
Instead of carbon brushes, I started using copper wires, they burn out faster, but give a larger spark.
I get even more spark using tin soldering wire.
My question is what can be used to cover the commutator and which brushes (tin, copper, etc) should be used to generate the largest spark?
To do this, I covered the contacts on the commutator with a marker to make the contact worse. Supplied 19.8 V from laptop charging, serial connection of electromagnets with rotor windings, 15 ohms. The current was about 1A.
After about 20 minutes of rotation of the engine, the brushes were worn off.
Instead of carbon brushes, I started using copper wires, they burn out faster, but give a larger spark.
I get even more spark using tin soldering wire.
My question is what can be used to cover the commutator and which brushes (tin, copper, etc) should be used to generate the largest spark?