MacIntoshCZ
Active Member
Hello everyone,
I am failing to get decent quality pcb. Biggest problem is that foil is not removed correctly. As you can see in image there are spots where you can see cuprexit layer, but honestly that was done by my finger. I am using negative photoresist film. Its old one and its really blue even before UV light exposure. I dont know what can cause this. Any ideas?
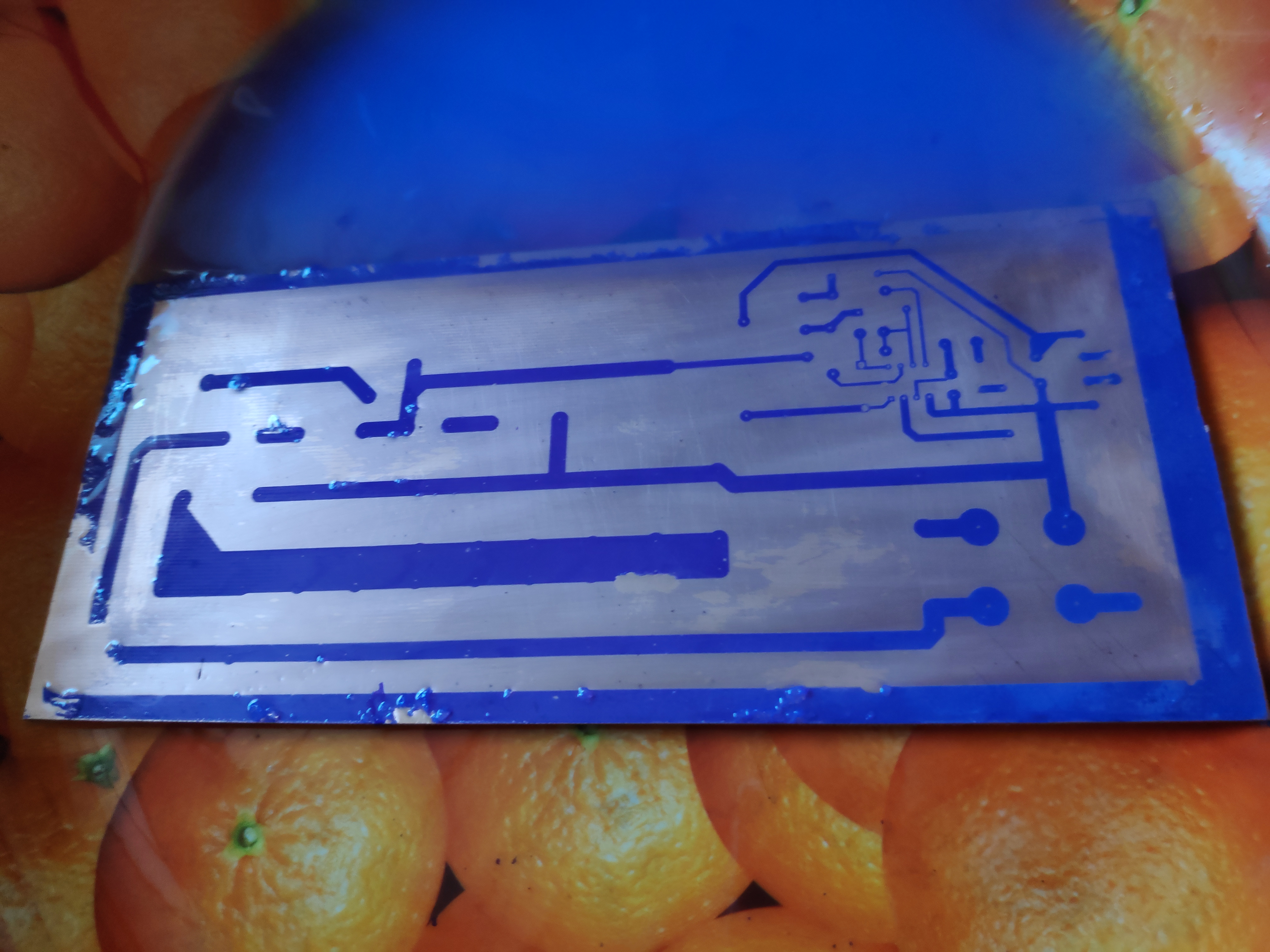

I am failing to get decent quality pcb. Biggest problem is that foil is not removed correctly. As you can see in image there are spots where you can see cuprexit layer, but honestly that was done by my finger. I am using negative photoresist film. Its old one and its really blue even before UV light exposure. I dont know what can cause this. Any ideas?

