lord loh.
Member
I have been reading all previous threads on Battery Charging circuits and was wondering that in a basic charger circuit which requires current to charge, how do I maintain the current,
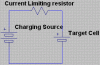
If I place a current limiting resistor, shall not form a potential divider with the cell and give a low voltage drop across the charging cell?
I am assuming that I can consider the resistance of the cell large as I am connecting it parallel to the charging source. The current form the target cell shall oppose the current from the source cell. And as the target cell charges, it's voltage level shall incerease and so shall it's current delivering capacity. And it's "resistance" shall further increase. Is my assumtion correct?
So do I need to decrease the voltage output across the terminals of the source as the target battery charges?
And how much current would a NiCd cell need for fast charge(1/4 rated?) and trickle charge(1/10 rated?)?
Please guide me...
Thank you.
[Oh! sorry, I wanted to post this in the general electronics Chat. Can a moderator move it there for me? Thank you.]
If I place a current limiting resistor, shall not form a potential divider with the cell and give a low voltage drop across the charging cell?
I am assuming that I can consider the resistance of the cell large as I am connecting it parallel to the charging source. The current form the target cell shall oppose the current from the source cell. And as the target cell charges, it's voltage level shall incerease and so shall it's current delivering capacity. And it's "resistance" shall further increase. Is my assumtion correct?
So do I need to decrease the voltage output across the terminals of the source as the target battery charges?
And how much current would a NiCd cell need for fast charge(1/4 rated?) and trickle charge(1/10 rated?)?
Please guide me...
Thank you.
[Oh! sorry, I wanted to post this in the general electronics Chat. Can a moderator move it there for me? Thank you.]